HPV vaccines
Statistics indicate that oral HPV infections decreased by 85% in young people who received as little as one dose of HPV vaccine.
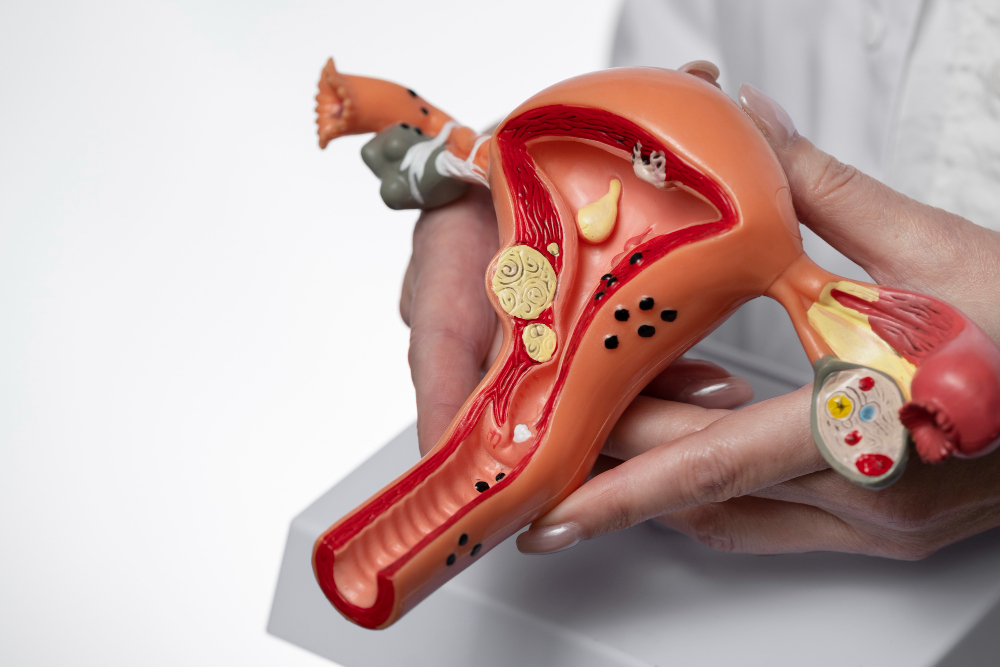
About 90% of cervical cancer cases are related to the human papillomavirus, which is one of the most frequent sexually transmitted infections today.
Approximately 300 million doses of HPV vaccine have been administered worldwide since its approval in 2006. Immunizations in children and young people are aimed at minimizing cases of cervical cancer and other pathologies caused by the virus.
What disease does the HPV vaccine prevent?
HPV does not only affect the cervix; it is known that the virus can influence the development of other types of cancers such as: mouth, throat, penis, anus, vagina and vulva.
Inoculation with HPV vaccines has shown close to 100% protection against infections, genital warts and certain precancerous lesions caused by human papilloma; for this and more, it is widely recommended by physicians and experts around the world.
Types of HPV vaccines
HPV has about 15 proven high oncogenic risk genotypes, these are: HPV 16, 18, 31, 33, 35, 35, 45, 51, 52, 56, 56, 58, 59, 59, 68, 73, 82. Of these, it is estimated that 70% of cervical cancers are caused by genotypes 16 and 18.
To date, there are three commercial vaccines for HPV prevention:
Cervarix®: Bivalent vaccine that provides protection against genotypes 16 and 18.
Gardasil®: Quadrivalent vaccine which protects against the most common genotypes (6, 11, 16 and 18).
Gardasil® 9: Boosted and broadly effective vaccine that protects against infection with 9 types of HPV (6, 11, 16, 18, 31, 33, 45, 52 and 58).
Gardasil®.
Gardasil® is a vaccine indicated for the prevention of diseases associated with human papillomavirus, especially genotypes 6, 11, 16 and 18. It is important to note that inoculation with Gardasil® does not work as a treatment in people who have previously contracted HPV.
Composition
Highly purified non-infectious protein of human papillomavirus type 6.
Highly purified non-infectious protein of human papillomavirus type 11.
Highly purified non-infectious protein of human papillomavirus type 16.
Highly purified non-infectious protein of human papillomavirus type 18.
Amorphous aluminum hydroxyphosphate sulfate (adjuvant).
Sodium Chloride.
Histidine.
Polysorbate 80.
Borax.
Water.
Recommendations
Gardasil® has proven to be an effective drug in the prevention of HPV 6, 11, 16 and 18 related diseases, benefiting women between 16 and 45 years of age and men from 16 to 26 years of age.
To increase the efficacy of the vaccine and stimulate the production of antibodies against the virus, administration of the vaccine is recommended in children and adolescents between 9 and 15 years of age.
Gardasil® 9
It could be said that Gardasil® 9 is an improved version of Gardasil®. This vaccine, in addition to preventing people from contracting the 2 genotypes most at risk for cervical cancer, also acts against oncogenic types 6 and 11 (which cause 90% of genital warts) and 5 other genotypes responsible for cancer.
Composition
Highly purified non-infectious protein of human papillomavirus type 6.
Highly purified non-infectious protein of human papillomavirus type 11.
Highly purified non-infectious protein of human papillomavirus type 16.
Highly purified non-infectious protein of human papillomavirus type 18.
Highly purified non-infectious protein of human papillomavirus type 31.
Highly purified non-infectious protein of human papillomavirus type 33.
Highly purified non-infectious protein of human papillomavirus type 45.
Highly purified non-infectious protein of human papillomavirus type 52.
Highly purified non-infectious protein of human papillomavirus type 58.
Amorphous aluminum hydroxyphosphate sulfate (adjuvant).
Sodium Chloride.
Histidine.
Polysorbate 80.
Borax.
Water.
Recommendations
Gardasil® 9 has been tested in women from 9 to 45 years of age and men from 9 to 26 years of age, showing satisfactory results in the protection against the 9 most predominant HPV genotypes.
Inoculation with Gardasil® 9 is completely safe in boys and girls from 9 years of age, with a 3-dose vaccination schedule.
Cervarix
This vaccine acts directly to protect against HPV genotypes 16 and 18, the main precursors of cervical cancer. Cervarix® is a preventive drug, so it does not cause any curative effect in people infected with HPV.
Composition
Highly purified non-infectious protein of human papillomavirus type 16.
Highly purified non-infectious protein of human papillomavirus type 18.
Monophosphoryl Lipid A.
Aluminum hydroxide.
Sodium chloride.
Sodium dihydrogen phosphate dihydrate.
Water.
Recommendations
Cervarix® is indicated for protection against HPV in boys and girls from 9 years of age and older.
HPV vaccine in girls
Since its approval in 2006, at least 70 countries around the world have included the HPV vaccine as part of the national vaccination schedule. Australia was one of the first countries to join this initiative, starting in 2007 with a school vaccination plan for girls between 12 and 13 years of age. In Spain, since 2008, 12-year-olds have been vaccinated free of charge.
Although we must admit that this topic is still controversial, multiple studies have proven that HPV vaccines are one of the safest and most effective methods to prevent this and other diseases in the future.
Do all adults need to be vaccinated against HPV?
Generally, HPV vaccination is advised at an early age (9-12 years) to increase the immune response to the virus and maximize the benefits before becoming sexually active. However, young adults who have not been vaccinated can be vaccinated up to age 26.
Individuals over the age of 27 who are interested in HPV vaccines should discuss with their physician, as factors such as age and exposure to the virus are important determinants of vaccine efficacy.

Frequently Asked Questions - HPV Vaccine
Although HPV vaccines may sometimes offer a minimal percentage of protection to the infected person, you should be aware that HPV inoculation does not have the ability to cure existing infections.
Yes, vaccinated women should continue to have regular check-ups in order to rule out infections or other pathologies not related to HPV vaccination.
It is recommended that pregnant women wait until after giving birth to receive the HPV vaccine. If you suspect a possible pregnancy, confirm the diagnosis before starting the vaccination schedule.
Some private insurers cover HPV vaccines in full, while others cover them on a reimbursement basis, so please discuss this information directly with your health insurance advisor.
We cannot say that there is an established maximum age for HPV vaccination. However, we should mention that the aim of the vaccine is to protect people from the human papillomavirus from the first sexual act; that is why the efficacy is higher in children and adolescents.
Children who start vaccination before 15 years of age can achieve full protection with only 2 doses. On the other hand, those who receive the vaccination after 15 years of age will require the full 3-dose vaccination schedule.
Since their approval, HPV vaccines have undergone hundreds of safety and efficacy tests, and to date, no serious side effects or adverse events have been reported.
Typically in these situations, patients experience the common pain of an injection in the arm or brief fainting spells due to nerves.
